CRB on Pharma 4.0™
The technology and advancements of Industry 4.0 are driving biotech forward, but only for those who understand its use cases, and know where and how to apply it. For others, it's only a buzzword. Our SMEs are here to ensure you can capture true efficiency and the growing value of 4.0-enabled operations.
Meet our 4.0 experts

Ryan Thompson
Ryan brings more than 17 years of experience successfully leading companies and projects through their digital transformation. He leverages a unique combination of technical expertise, strong leadership, and clear communication to drive meaningful, scalable Industry 4.0 strategies and roadmaps.

Yvonne Duckworth, PE
Yvonne is a registered automation engineer with 30 years of technical design and project leadership experience within the pharmaceutical and biotech industries. Her expertise includes developing overall control system strategy, instrumentation design, process control system design and much more.

Matt Edwards
Matt leads the virtual design and construction (VDC) team at CRB which includes people, processes and tools around CRB’s global offices. He works with design and construction teams to implement VDC technologies, workflows and standards to positively impact our client’s projects.

Riju Saini, PhD
Fellow - Simulation, Modeling, CFD
Riju helps clients by driving growth, reducing cost, improving operations and creating solutions from complex business and technical problems. He is an expert in conceptual process design, thermodynamics, steady state and dynamic process simulation, consequence and risk analysis and much more.
Our digitalization team is excited to see you at ISPE 2024!
Catch us on stage at Annual Meeting:
- Transformational Efficiency in Next-Gen Facilities
- Yvonne Duckworth, CRB with Jim Weidner, Amgen
- Monday, Oct 14th @ 1300
Reducing Cost of Goods (CoGs)
How are YOU using modern technology in your operations?
Applying Industry 4.0:
Use Cases for Biopharma Efficiency
With Yvonne Duckworth & Ryan Thompson
How are you increasing productivity and efficiency in your operations? Hear real-world examples of how the industry and your peers are using AI, ML, VR and Industry 4.0 to improve operations in this on-demand, 45-minute webinar.
Industry guides and references
Regulatory guidance:
FDA: Emerging Technologies Program
FDA: Digital Health Advisory Committee
EMA: Quality Innovation Group / QIG – Listen & Learn focus group meetings
Industry guidance:
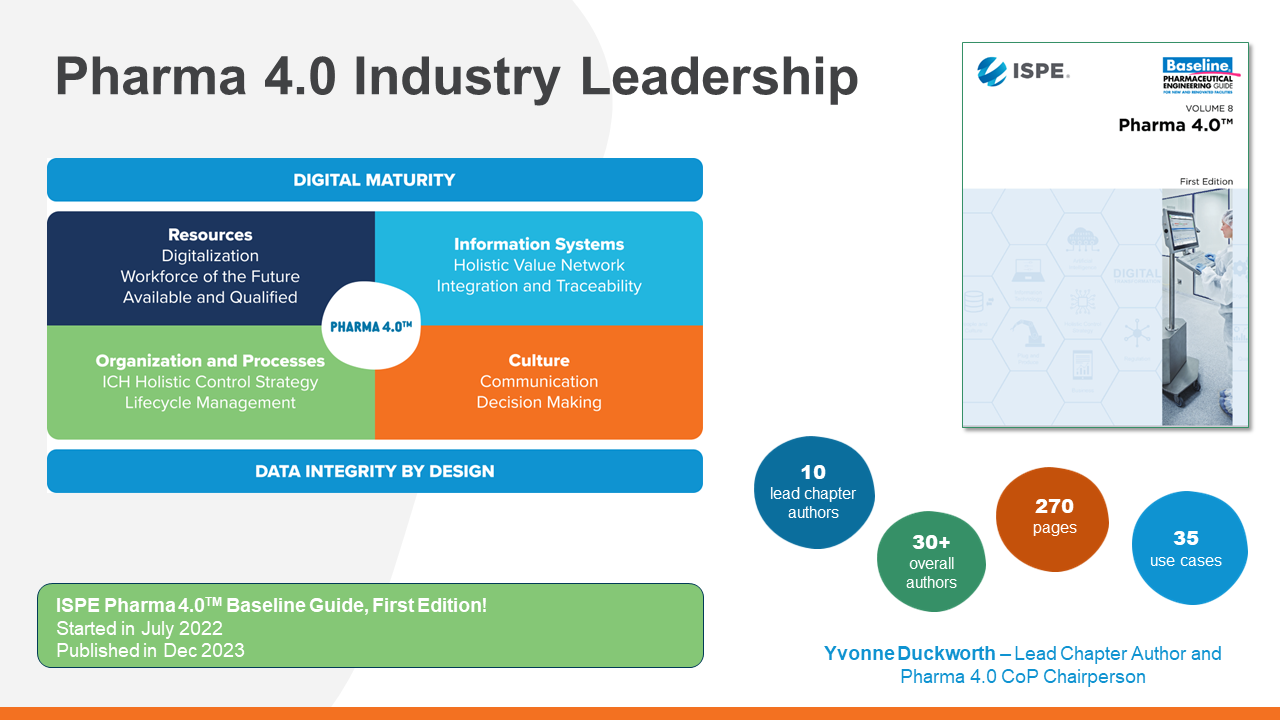
ISPE: Pharma 4.0TM Baseline Guide
SIRI: Smart Industry Readiness Index
BioPhorum: Digital Plant Maturity Model 3.0
Industry data
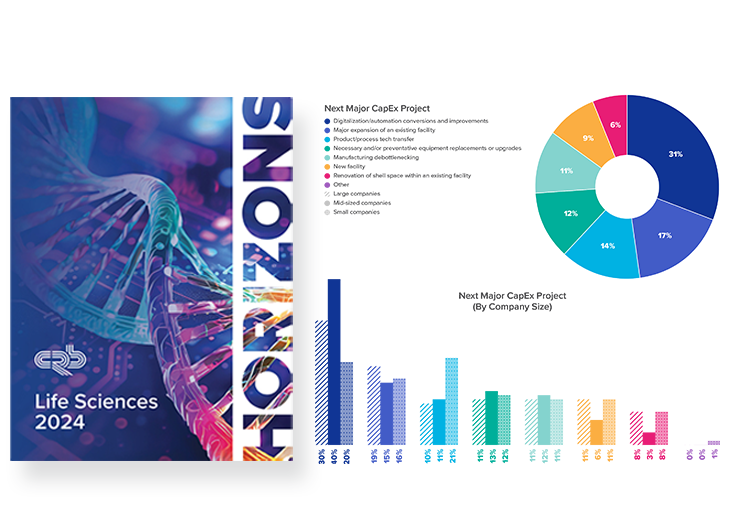
2024 Horizons: Life Sciences report
How much of biopharma's CapEx is going to digitalization projects? And how exactly is Industry 4.0 being used in labs?
Based on survey responses from hundreds of industry leaders, our new data and report go deep into these questions and other critical trends driving the industry.
Need help moving your 4.0 project forward?
We can assist you in planning for advanced manufacturing opportunities within the design of your facility, optimizing your processes, creating an AI strategy, or helping bridge the data gap between R&D and commercialization.
CRB’s experts work with you to:
- Identify high-value, low-risk use cases for automation within your facility
- Develop digital transformation maturity assessments and roadmaps
- By acting as an Owner Representative from requirements development to completion
- Leverage digital strategies to reduce costs while scaling your business or process
- Develop models, simulations and digital twins to find answers to your most challenging problems
Let's talk about your project and goals.
Industry 4.0 Insights

Intro to Pharma 4.0™ and facility digitalization
The Pharma 4.0™ operating model accounts for how to adopt this technology into the heavily regulated pharmaceutical industry, and how to do so safety and securely.

How the automation pyramid enables commercial-scale ATMP manufacturing
Use the automation pyramid as a roadmap to navigate the transition from lab-scale ATMP manufacturing to a cGMP facility that’s compliant and optimized.

Augmented reality and virtual reality improve project delivery
Improve efficiency throughout your project. Leveraging virtual reality (VR) and augmented reality (AR) on a project site helps to eliminate bottlenecks, improve collaboration, and enable lean delivery.
Scaling cell and gene therapy operations with Industry 4.0
On-demand webinar
Cell and gene therapies are unlocking a remarkable new landscape of medicine. But the reality is that the science and production are complex and expensive, limiting patient access. Integrating an Industry 4.0 strategy can enable a new trajectory for scaling and navigating the CGT commercial manufacturing process.
Together, Walters and Thompson look at practical and accessible ways to scale production for personalized medicines with data, digitization, robotics and process closure
Where are you on your digitalization projects?
Meet your goals by building a strong foundation. Consider these questions when getting started with 4.0 projects:
- What am I trying to achieve?
- Where am I now in respect to that goal?
- What technologies are available?
- What are my peers doing?
- What are the costs?
- Initial investment
- Annual or recurring
- How will it impact design?
- Who are the key vendors?
- Who are the key integrators that can implement the technology?
- What are the risks in terms of manufacturing, product safety, and cybersecurity?
- What is the impact on my workforce?
- Is it possible to set up a test-and-learn sandbox at an existing site to reduce the operational risks and costs for a new facility?
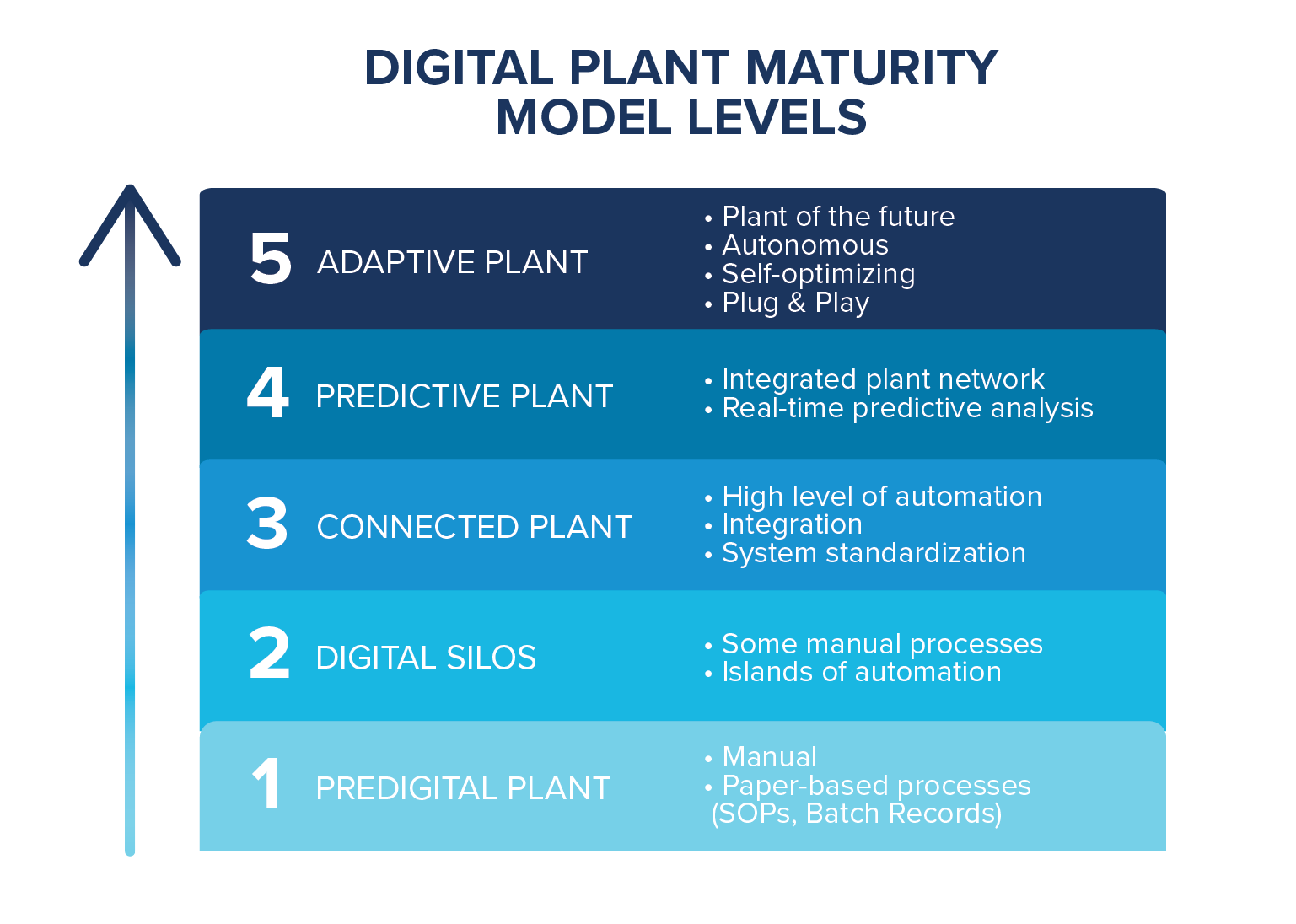
Start with the Smart Industry Readiness Index (SIRI) or a Digital Plant Maturity Model (DPMM) assessment and project roadmap. A biopharma-specific DPMM was developed by BioPhorum to help companies accurately identify where they are to plan project next steps from a place of knowledge and insight.
Once you understand where you are in relation to your goal, you can map out the necessary steps to get you there in a project-specific 4.0 roadmap.